TOMZ Corporation is looking for a motivated Associate Project Manager to join our organization. A leader in manufacturing of devices and components for major medical device companies, TOMZ offers competitive compensation and excellent benefits, including 401k, health/dental, vision, and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and is certified to ISO 13485.
Summary of Position
Reporting to the Chief Commercial Officer, this position is responsible for assisting the planning, execution, monitoring, and completion of projects with cross-functional teams to successfully managing the development of new and existing Projects. With assistance utilizing project management and system tools to track projects deliverables.
Essential Functions
Assist all functional stakeholders to manage and coordinate activities related to the development, documentation and validation of medical devices utilizing TOMZ project tracker.
- Guidance from Team Leader, support members of the organization understand their roles and responsibilities. Motivate and inspire each other to achieve project goals and objectives.
- the implementation of Change Management process to effectively manage changes to project scope, schedule, and objectives defined by the customer while minimizing disruptions. This requires assessing impacts, obtaining approvals, and communicating changes to relevant stakeholders.
- With guidance, generate and maintain project plans, schedules, task lists and project risk registers that enable the team to execute project activities.
- Coordinate activities across different functional groups within the company, customers, and suppliers to ensure successful completion of project goals and milestones.
- Additional responsibilities as outlined in full job description.
Qualifications:
Education/Experience
- 4-Year degree or 2+ years manufacturing/equivalent of directly transferrable industry work experience and prior experience with project management
- Experience in a regulated engineering/manufacturing environment with mechanical, tool design, and manufacturing processes preferred (preferably in a Medical Device)
TOMZ Corporation is looking for a motivated Associate Project Manager to join our organization. A leader in manufacturing of devices and components for major medical device companies, TOMZ offers comp...
TOMZ Corporation is looking for a Quality Engineering Manager to join our organization. A leader in manufacturing of devices and components for major medical device companies, TOMZ offers competitive compensation and excellent benefits, including 401k, health/dental, vision and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and is certified to ISO 13485.
Summary of Position
This position will be responsible for the Leadership and management of site-level Quality Engineering personnel and resources to ensure adherence and compliance to TOMZ's Quality Management System.
Essential Functions
- This position may have direct-reporting responsibilities for daily activities of Quality personnel.
- Designated Site-level Management Representative for assurance of compliance to the TOMZ Quality Management System.
- Drive and support QMS initiatives and continuous improvement activities.
- Support establishment and maintenance of site regulatory registrations (ISO 13485, FDA, etc.).
- Provide organizational Quality Leadership, coaching, mentoring and guidance to all Quality Engineering, as well as cross-functional teams.
- Work closely with Engineering and Operations teams to identify and drive actions that reduce the Cost of Poor Quality.
- Drive integration of new manufacturing capabilities, new test methods and innovation in technology, cost/labor reduction opportunities, product transfers, design transfers and sustained manufacturing customers.
- Additional responsibilities as outlined in the full job description.
Qualifications:
Education
- Minimum 4-Year degree or equivalent of directly transferrable industry work experience (Engineering or Quality discipline preferred)
Experience
- of 1-3 years Leadership/Supervisory-level experience, with budget and decision-making authority/responsibilities.
- of 5 years of experience in a regulated manufacturing environment.
Additional qualifications as outlined in full job description.
TOMZ Corporation is looking for a Quality Engineering Manager to join our organization. A leader in manufacturing of devices and components for major medical device companies, TOMZ offers competitive ...
TOMZ Corporation is looking for a Plant Supervisor (weekend shift) to join our organization. A leader in manufacturing of devices and components for major medical device companies, TOMZ offers competitive compensation and excellent benefits, including 401k, health/dental, vision and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and is certified to ISO 13485.
Summary of Position
Supervises production team in assigned department(s). Improve safety, quality, delivery, productivity, and cost. Increase team skills and capabilities. Continuously improves level of associate satisfaction while improving efficiency.
Essential Functions
- Safe operation of all production processes. Identifies improvements needed and leads project for implementation. Continually monitors area and solicits feedback from associates. Enforce all safety rules and regulations. Is key coordinator/communicator for at least one safety training program for the entire organization.
- Supervises team members. Ensures effective employee relations. Resolves issues through problem resolution. Confers with management to resolve complex associate issues.
- Interviews, selects, orients, trains, conduct performance appraisals, performance development, schedules and approves hours.
- Provides leadership for associate relations through effective communications, coaching, training and development.
- Executes operational plan to enhance profitability, productivity and efficiency throughout assigned production department(s)
- Additional responsibilities as outlined in full job description.
Qualifications:
Education/Experience
- High school diploma, GED, or equivalent directly transferrable work experience (Manufacturing or Engineering discipline preferred).
- from a technical school program strongly preferred.
- degree or equivalent in Business Operations or closely related field preferred OR directly transferrable industry work experience.
Job Features
TOMZ Corporation is looking for a Plant Supervisor (weekend shift) to join our organization. A leader in manufacturing of devices and components for major medical device companies, TOMZ offers competi...
TOMZ Corporation is looking for a Plant Supervisor (night shift) to join our organization. A leader in manufacturing of devices and components for major medical device companies, TOMZ offers competitive compensation and excellent benefits, including 401k, health/dental, vision and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and is certified to ISO 13485.
Summary of Position
Supervises production team in assigned department(s). Improve safety, quality, delivery, productivity, and cost. Increase team skills and capabilities. Continuously improves level of associate satisfaction while improving efficiency.
Essential Functions
- Safe operation of all production processes. Identifies improvements needed and leads project for implementation. Continually monitors area and solicits feedback from associates. Enforce all safety rules and regulations. Is key coordinator/communicator for at least one safety training program for the entire organization.
- Supervises team members. Ensures effective employee relations. Resolves issues through problem resolution. Confers with management to resolve complex associate issues.
- Interviews, selects, orients, trains, conduct performance appraisals, performance development, schedules and approves hours.
- Provides leadership for associate relations through effective communications, coaching, training and development.
- Executes operational plan to enhance profitability, productivity and efficiency throughout assigned production department(s)
- Additional responsibilities as outlined in full job description.
Qualifications:
Education/Experience
- High school diploma, GED, or equivalent directly transferrable work experience (Manufacturing or Engineering discipline preferred).
- from a technical school program strongly preferred.
- degree or equivalent in Business Operations or closely related field preferred OR directly transferrable industry work experience.
TOMZ Corporation is looking for a Plant Supervisor (night shift) to join our organization. A leader in manufacturing of devices and components for major medical device companies, TOMZ offers competiti...
TOMZ Corporation is looking for a motivated Application Engineer to join our organization. A leader in manufacturing of devices and components for major medical device companies, TOMZ offers competitive compensation and excellent benefits. Those include 401k, health/dental, vision, and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and certified to ISO 13485.
Summary of Position
Support the organization’s Turning, Milling and Grinding operations of complex medical devices through new development projects as well as optimization of existing legacy programs. Work in cross functional teams to drive continuous improvement and ensure production objectives are met; including but not limited to programming for new product development, optimization of legacy programs for improved throughput, standardization of programs to current best practices, and research and implementation of new tooling and process technology. TOMZ is a fast-paced work environment.
Essential Functions
Duties and responsibilities include, but are not limited to:
- Ability to program various pieces of CNC equipment across multiple sites as directed to support critical and/or high-value projects milling, turning, and grinding, Multi spindle, Multi Axis and Swiss style programs for manufacture of medical devices- implants and instruments.
- Analyze drawings, design models, and sketches to determine dimensions and configuration of cuts, selection of cutting tools, machine speeds, and feed rates according to shop processes, customer specifications, and machine capabilities.
- Actively participate in process development projects and DFM with internal and external teams
- Revise programs to eliminate instruction errors, omissions, and update programs with new technology/methods to improve efficiency; meet customer delivery dates.
- Coordinate with Quality Engineering to help facilitate the development of Process Control Plans, Manufacturing Inspection Plans, and gaging/fixturing requirements.
- Machine set-up skills required to accomplish 1st article parts and short run NPI products. With an occasional small production run if needed.
- Design fixtures for post processing operations.
- Additional responsibilities as outlined in full job description.
Qualifications:
Education
- Associate degree in technical or scientific field or equivalent work experience.
Experience
- Minimum of 5 years’ experience of programming and machine set-up in a medical device manufacturing environment or similar.
TOMZ Corporation is looking for a motivated Application Engineer to join our organization. A leader in manufacturing of devices and components for major medical device companies, TOMZ offers competiti...
Our Mission
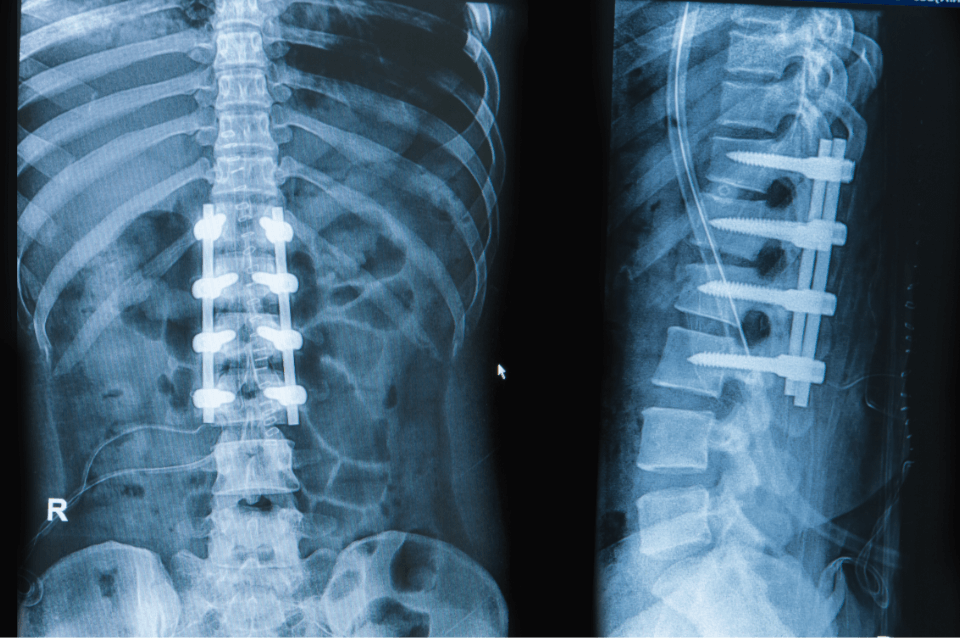
To enhance the lives of patients by sustainably manufacturing innovative medical devices for leading OEMs around the globe.


We Manufacture Medical Devices
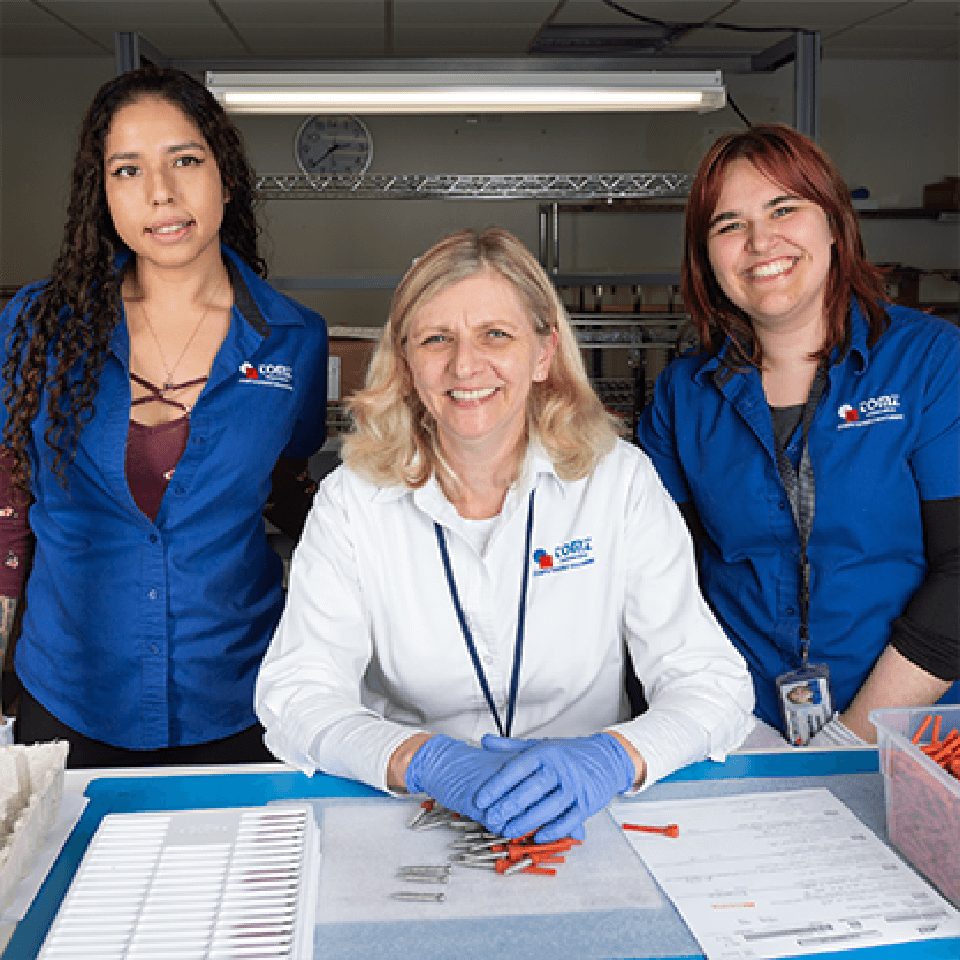
We specialize in manufacturing the best medical device implants for medical teams and surgeons around the world. Most of our team will tell you that the work is very fulfilling because we help people live better lives.
Company Values & Culture
Our DNA is as unique as everyone that works here.
Put Patients First
Embrace Diversity

Learn & Grow

Reward Great Effort
Why Work at TOMZ?
We are large enough to offer stability and great benefits, yet small enough to recognize you as an individual and not just “another employee.”
Multiple departments from facilities management to engineering allows you to explore different career paths.
The medical devices industry is experiencing explosive growth with no signs of stopping any time soon.
We have completed our 8th expansion which includes amenities such as locker rooms, showers, an expanded cafeteria, and more.
We have a deep bench of knowledgeable pros to help you.
Everything from 401K to top-of-the-line healthcare, life insurance, and more!
What Our Team Members Have to Say
Playlist
Benefits
401(k) Retirement Plan
We will match $0.50 on the dollar up to an 8% contribution. Work with us, retire with us.
Overtime Available
Opportunities to earn overtime hours are available as needed.
Paid Holidays
9 paid holidays AND enjoy a half-day the work day before the paid holiday.
Paid Vacation
Paid vacation starts accruing from the day you start.
Referral Bonus
All employees are eligible to earn a $3,500 referral bonus!
Health Insurance Plans
Enjoy a low employee co-pay with high contributions towards your plan.
Dental Insurance Plans
Yes - we have a great dental plan as well!
Annual Bonus
Production teams achieving set performance goals receive an annual performance bonus.

